A reaction starting from reactants that change into products must pass through an unstable state at the highest energy level; this is called a transition state. A certain amount of energy is required to overcome the energy barrier, and this energy, called activation energy, is represented by ΔG‡. The magnitude of ΔG‡ determines the rate of a chemical reaction. When ΔG‡ increases, the reaction rate decreases, since more energy is required to pass through the energy barrier. The double dagger (‡) symbol is used when a reaction involves transition states. This is different from the free energy change (ΔG) in the previous section. They are illustrated in Figure 1.2 with combustion as an example:
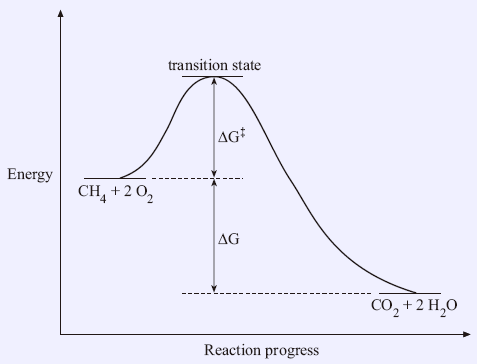
In Figure 1.2, the x-axis is called reaction progress, although sometimes it may be written as reaction coordinate, while the y-axis is the energy.
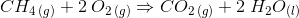
There are many reactions that take place in more than one step. They involve the formation of reactive intermediates. These reactions can be considered multistep reactions. For example, in the addition of hydrogen chloride to 2-methylpropene:
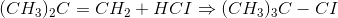
This can be regarded as a multistep reaction because it involves the following two steps:
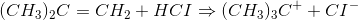
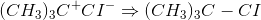
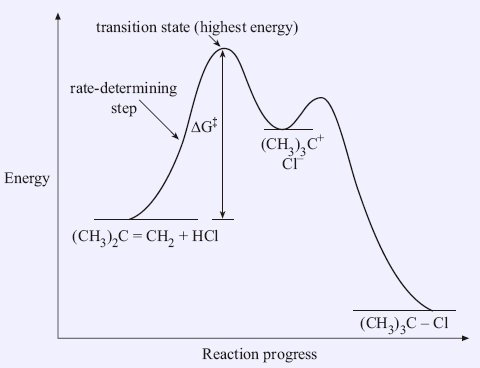
Each step has its own reaction rate and transition state. The free-energy diagram is shown in Figure 1.3. For multistep reactions, the slowest step is called rate-determiningstep (or rate-limiting step). The reaction rate of the overall reaction is equal to the rate of rate-determining step.
In the example shown in Figure 1.3, there are two transition states. The rate-determining step is the one with the transition state of highest free-energy.
- 19776 reads






