The rate of a chemical reaction can be defined as the number of starting molecules converted into product in a given time. When a reaction can give more than one possible product, i.e.more than one reaction are occurring simultaneously, these reactions are in competition. The one predominating occurs more rapidly than the others, and hence it reacts preferentially in the reactions. In order to determine which reaction is the most feasible, the term Gibbs free energy change (ΔG) must be considered in addition to the chemical kinetics. This is why the term reactivity must include both kinetic and thermodynamic factors, as you learned in the previous section. In fact, ΔG refers to whether a chemical change will take place (related to thermodynamics), while kinetics just describe how fast the reaction is.
For free energy change:
ΔG = ΔH – TΔS
(where ΔG: free energy change; ΔH: enthalpy change; T: temperature; ΔS: entropy change)
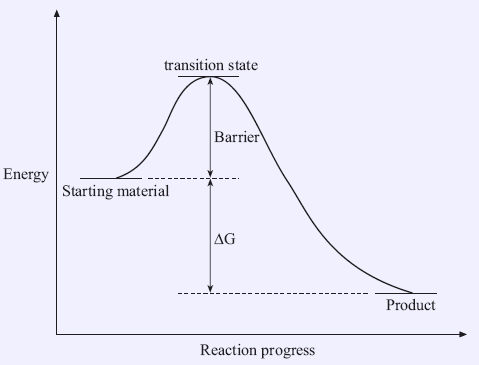
Both kinetic and thermodynamic effects should be considered. Although conditions for a reaction to occur should be favorable if both enthalpy and entropy are favorable (i.e. a very positive change in entropy and the reaction is highly exothermic), the reaction still will not happen if the reaction rate is too slow. Hence, an exothermic reaction does not necessarily lead to a fast reaction. This is because there is a barrier to the chemical reactions (Figure 1.1). Again, reaction rates are not determined by the free energy change (ΔG) between the starting material and the product; they are determined by the height of barrier between the starting material and the transition state.
If a reactant can give two different products via different reaction pathways, the more thermodynamically stable one will usually form, but there are exceptional cases in which the reaction is considered to be kinetically-controlled. In such cases, the less thermodynamically stable product will result in a faster reaction rate. The dichotomy of competition between thermodynamic and kinetic control reaction will be discussed in a later section.
- 2991 reads






