A gas expands according to the law,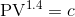 . Initially, the
pressure is 100 kPa when the volume is 1 m3. Write a script to compute the work done by the gas in expanding to three times its original volume 1
. Initially, the
pressure is 100 kPa when the volume is 1 m3. Write a script to compute the work done by the gas in expanding to three times its original volume 1
Recall that PV diagrams can be used to estimate the net work performed by a thermodynamic cycle, see Wikipedia 2 or we can use definite integral to compute the work done (WD) as follows:
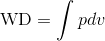
If we rearrange the expression pressure as a function of volume, we get:
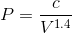
By considering the initial state, we can determine the value of c:
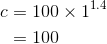
From the equation (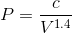 ) and the equation (
) and the equation (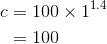 ) above, we can write:
) above, we can write:
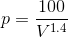
By inserting P (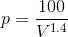 ) in WD (
) in WD (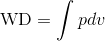 ), we get:
), we get:
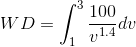
For MATLAB solution, we will consider P as a function of V (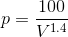 ) and WD
(
) and WD
(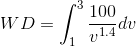 ). Now, let us apply the three-step approach we have used earlier:
). Now, let us apply the three-step approach we have used earlier:
- Initialize variable volume as a row vector, from 1 with increments of 0.001 to 3: v=1:0.001:3;
- Declare variable pressure as p=100./v.^1.4;
- Use the trapz function to calculate the work done, the output will be as follows:
WorkDone=trapz(v,p)
WorkDone =
88.9015
These steps can be combined in an m-file as follows:
clc
disp('A gas expands according to the law, pv^1.4=C')
disp('Initial pressure is 100 kPa when the volume is 1 m3')
disp('Compute the work done by the gas in expanding')
disp('To three times its original volume')
disp(' ') % Display blank line
v=1:.001:3; % Creating a row vector for volume, v
p=100./(v.^1.4); % Computing pressure for volume
WorkDone=trapz(v,p) % Integrating p*dv over 1 to 3 cubic meters
- 瀏覽次數:2023






