An atom is the smallest component of an element that retains all of the chemical properties of that element. For example, one hydrogen atom has all of the properties of the element hydrogen, such as it exists as a gas at room temperature, and it bonds with oxygen to create a water molecule. Hydrogen atoms cannot be broken down into anything smaller while still retaining the properties of hydrogen. If a hydrogen atom were broken down into subatomic particles, it would no longer have the properties of hydrogen.
At the most basic level, all organisms are made of a combination of elements. They contain atoms that combine together to form molecules. In multicellular organisms, such as animals, molecules can interact to form cells that combine to form tissues, which make up organs. These combinations continue until entire multicellular organisms are formed.
All atoms contain protons, electrons, and neutrons (Figure 2.2). The only exception is hydrogen (H), which is made of one proton and one electron. A proton is a positively charged particle that resides in the nucleus (the core of the atom) of an atom and has a mass of 1 and a charge of +1. An electron is a negatively charged particle that travels in the space around the nucleus. In other words, it resides outside of the nucleus. It has a negligible mass and has a charge of –1.
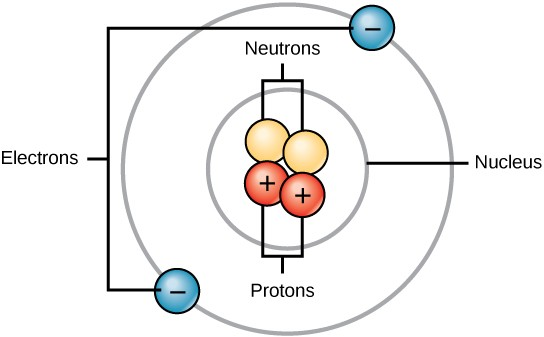
Neutrons, like protons, reside in the nucleus of an atom. They have a mass of 1 and no charge. The positive (protons) and negative (electrons) charges balance each other in a neutral atom, which has a net zero charge.
Because protons and neutrons each have a mass of 1, the mass of an atom is equal to the number of protons and neutrons of that atom. The number of electrons does not factor into the overall mass, because their mass is so small.
As stated earlier, each element has its own unique properties. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. The atomic numberof an element is equal to the number of protons that element contains. The mass number, or atomic mass, is the number of protons plus the number of neutrons of that element. Therefore, it is possible to determine the number of neutrons by subtracting the atomic number from the mass number.
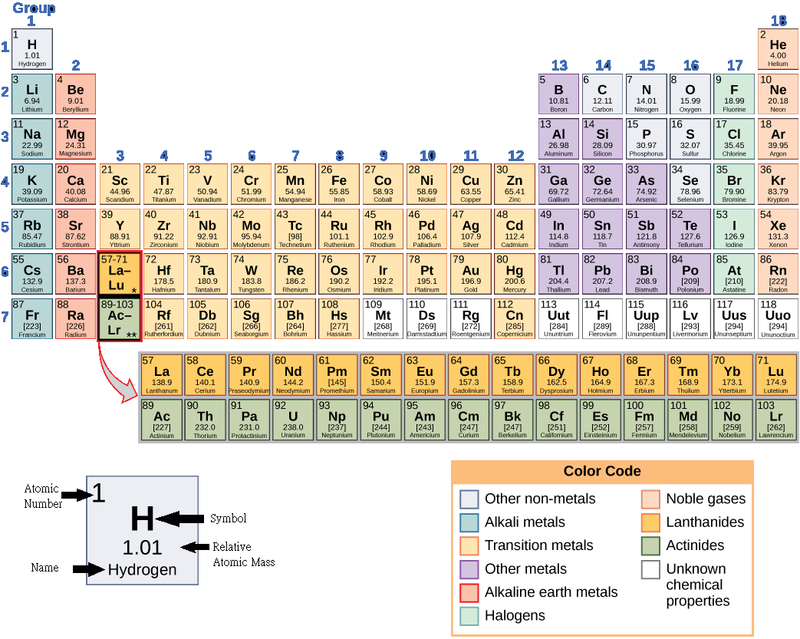
These numbers provide information about the elements and how they will react when combined. Different elements have different melting and boiling points, and are in different states (liquid, solid, or gas) at room temperature. They also combine in different ways. Some form specific types of bonds, whereas others do not. How they combine is based on the number of electrons present. Because of these characteristics, the elements are arranged into the periodictable of elements, a chart of the elements that includes the atomic number and relative atomic mass of each element. The periodic table also provides key information about the properties of elements (Figure 2.2)—often indicated by color-coding. The arrangement of the table also shows how the electrons in each element are organized and provides important details about how atoms will react with each other to form molecules.
Isotopes are different forms of the same element that have the same number of protons, but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon-12, the most common isotope of carbon, contains six protons and six neutrons. Therefore, it has a mass number of 12 (six protons and six neutrons) and an atomic number of 6 (which makes it carbon). Carbon-14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (six protons and eight neutrons) and an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more stable elements. These are called radioactive isotopes or radioisotopes.
Art Connection
How many neutrons do (K) potassium-39 and potassium-40 have, respectively?
Evolution In Action
Carbon Dating
Carbon-14 (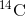 ) is a naturally occurring radioisotope that
is created in the atmosphere by cosmic rays. This is a continuous process, so more
) is a naturally occurring radioisotope that
is created in the atmosphere by cosmic rays. This is a continuous process, so more 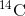 is always being created. As a living
organism develops, the relative level of
is always being created. As a living
organism develops, the relative level of 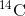 in its body is equal to the
concentration of
in its body is equal to the
concentration of  in the atmosphere. When an organism
dies, it is no longer ingesting
in the atmosphere. When an organism
dies, it is no longer ingesting 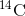 , so the ratio will decline.
, so the ratio will decline.
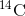 decays to
decays to 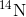 by a process called beta decay; it gives off energy in this slow process. After
approximately 5,730 years, only one-half of the starting concentration of
by a process called beta decay; it gives off energy in this slow process. After
approximately 5,730 years, only one-half of the starting concentration of 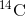 will have been converted to
will have been converted to
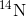 . The time it takes for half of the
original concentration of an isotope to decay to its more stable form is called its half-life. Because the half-life of
. The time it takes for half of the
original concentration of an isotope to decay to its more stable form is called its half-life. Because the half-life of 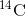 is long, it is used to age formerly
living objects, such as fossils. Using the ratio of the
is long, it is used to age formerly
living objects, such as fossils. Using the ratio of the 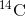 concentration found in an
object to the amount of
concentration found in an
object to the amount of 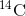 detected in the atmosphere, the
amount of the isotope that has not yet decayed can be determined. Based on this amount, the age of the fossil can be calculated to about 50,000 years (Figure 2.4). Isotopes with longer half-lives, such as potassium-40, are used to
calculate the ages of older fossils. Through the use of carbon dating, scientists can reconstruct the ecology and biogeography of organisms living within the past 50,000 years.
detected in the atmosphere, the
amount of the isotope that has not yet decayed can be determined. Based on this amount, the age of the fossil can be calculated to about 50,000 years (Figure 2.4). Isotopes with longer half-lives, such as potassium-40, are used to
calculate the ages of older fossils. Through the use of carbon dating, scientists can reconstruct the ecology and biogeography of organisms living within the past 50,000 years.

To learn more about atoms and isotopes, and how you can tell one isotope from another, visit this site (http://openstaxcollege.org/l/isotopes)and run the simulation.
- 5074 reads






