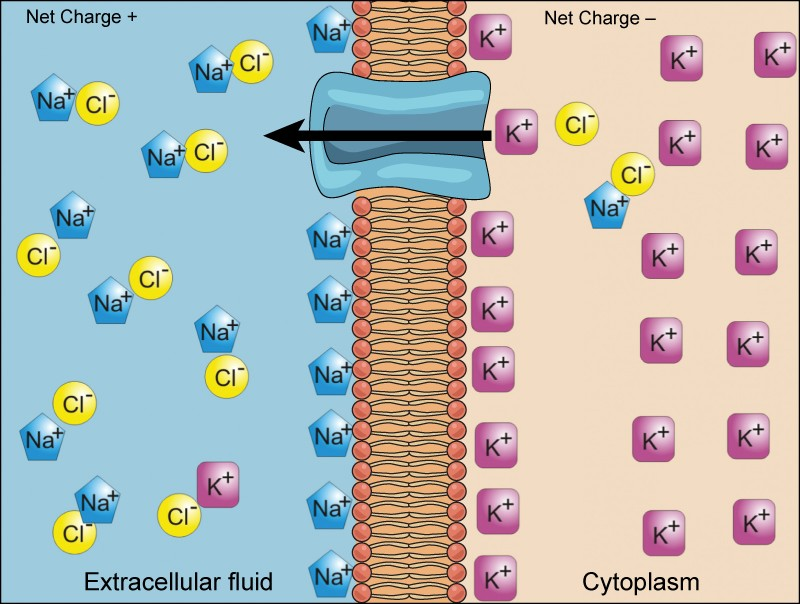
We have discussed simple concentration gradients—differential concentrations of a substance across a space or a membrane—but in living systems, gradients are more complex. Because cells contain proteins, most of which are negatively charged, and because ions move into and out of cells, there is an electrical gradient, a difference of charge, across the plasma membrane. The interior of living cells is electrically negative with respect to the extracellular fluid in which they are bathed; at the same time, cells have higher concentrations of potassium (K+) and lower concentrations of sodium (Na+) than does the extracellular fluid. Thus, in a living cell, the concentration gradient and electrical gradient of Na+ promotes diffusion of the ion into the cell, and the electrical gradient of Na+ (a positive ion) tends to drive it inward to the negatively charged interior. The situation is more complex, however, for other elements such as potassium. The electrical gradient of K+ promotes diffusion of the ion intothe cell, but the concentration gradient of K+ promotes diffusion outof the cell (Figure 3.24). The combined gradient that affects an ion is called its electrochemical gradient, and it is especially important to muscle and nerve cells.
- 3391 reads






