There are four types of bonds or interactions: ionic, covalent, hydrogen bonds, and van der Waals interactions. Ionic and covalent bonds are strong interactions that require a larger energy
input to break apart. When an element donates an electron from its outer shell, as in the sodium atom example above, a positive ion is formed. The element accepting the electron is now
negatively charged. Because positive and negative charges attract, these ions stay together and form an ionic bond, or a bond
between ions. The elements bond together with the electron from one element staying predominantly with the other element. When 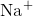 and
and 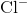 ions combine to produce
ions combine to produce 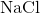 , an electron from a sodium atom stays with the other seven from the chlorine atom, and the sodium and chloride ions attract each
other in a lattice of ions with a net zero charge.
, an electron from a sodium atom stays with the other seven from the chlorine atom, and the sodium and chloride ions attract each
other in a lattice of ions with a net zero charge.
- 2942 reads






