Another familiar fermentation process is alcohol fermentation (Figure 4.17), which produces ethanol, an alcohol. The alcohol fermentation reaction is the following:
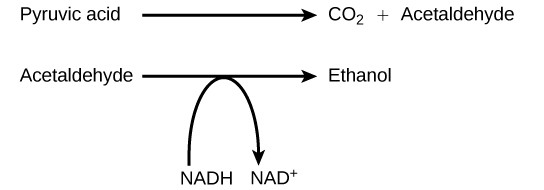
In the first reaction, a carboxyl group is removed from pyruvic acid, releasing carbon dioxide as a gas. The loss of carbon dioxide reduces the molecule by one carbon atom, making acetaldehyde.
The second reaction removes an electron from NADH, forming 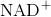 and producing ethanol from the acetaldehyde, which accepts the electron. The fermentation of pyruvic acid by yeast produces the
ethanol found in alcoholic beverages (Figure 4.18 ). If the carbon
dioxide produced by the reaction is not vented from the fermentation chamber, for example in beer and sparkling wines, it remains dissolved in the medium until the pressure is released. Ethanol
above 12 percent is toxic to yeast, so natural levels of alcohol in wine occur at a maximum of 12 percent.
and producing ethanol from the acetaldehyde, which accepts the electron. The fermentation of pyruvic acid by yeast produces the
ethanol found in alcoholic beverages (Figure 4.18 ). If the carbon
dioxide produced by the reaction is not vented from the fermentation chamber, for example in beer and sparkling wines, it remains dissolved in the medium until the pressure is released. Ethanol
above 12 percent is toxic to yeast, so natural levels of alcohol in wine occur at a maximum of 12 percent.

- 3362 reads






