Learning Objectives
By the end of this section, you will be able to:
- Explain what metabolic pathways are
- State the first and second laws of thermodynamics
- Explain the difference between kinetic and potential energy
- Describe endergonic and exergonic reactions
- Discuss how enzymes function as molecular catalysts
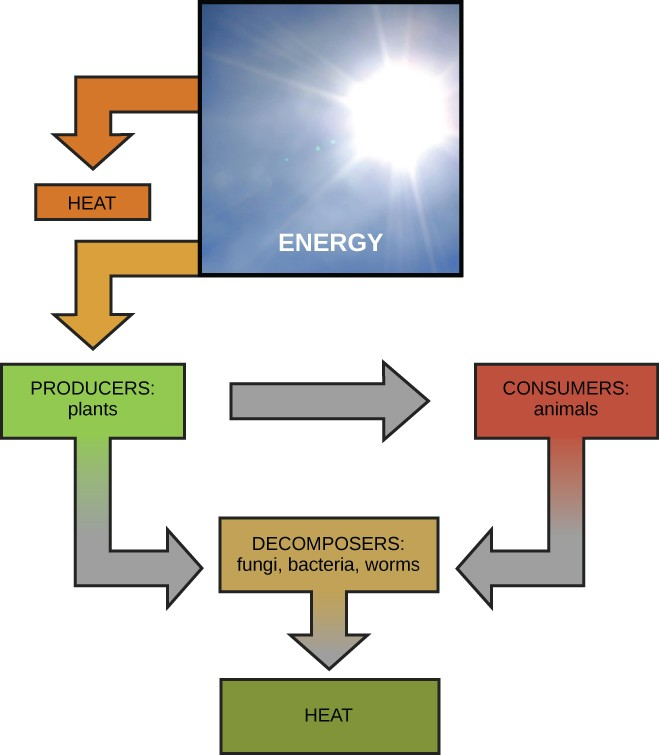
Scientists use the term bioenergetics to describe the concept of energy flow (Figure 4.2) through living systems, such as cells. Cellular processes such as the building and breaking down of complex molecules occur through stepwise chemical reactions. Some of these chemical reactions are spontaneous and release energy, whereas others require energy to proceed. Just as living things must continually consume food to replenish their energy supplies, cells must continually produce more energy to replenish that used by the many energy-requiring chemical reactions that constantly take place. Together, all of the chemical reactions that take place inside cells, including those that consume or generate energy, are referred to as the cell’s metabolism.
- 5052 reads






