Available under Creative Commons-ShareAlike 4.0 International License.
Two atoms will interact via electrical forces between their protons and electrons. To put them at a distance  from one another (measured from nucleus to nucleus), a certain amount of energy
from one another (measured from nucleus to nucleus), a certain amount of energy 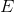 is required, and the minimum energy occurs when the atoms are in equilibrium,
forming a molecule. Often a fairly good approximation to the energy is the Lennard-Jones expression
is required, and the minimum energy occurs when the atoms are in equilibrium,
forming a molecule. Often a fairly good approximation to the energy is the Lennard-Jones expression
 from one another (measured from nucleus to nucleus), a certain amount of energy
from one another (measured from nucleus to nucleus), a certain amount of energy 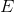 is required, and the minimum energy occurs when the atoms are in equilibrium,
forming a molecule. Often a fairly good approximation to the energy is the Lennard-Jones expression
is required, and the minimum energy occurs when the atoms are in equilibrium,
forming a molecule. Often a fairly good approximation to the energy is the Lennard-Jones expression
![E(r)=k\left [ \left ( \frac{a}{r} \right )^{12}-2\left ( \frac{a}{r} \right )^6 \right ]](/system/files/resource/33/33298/33786/media/eqn-img_42.gif)
where 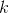 and
and  are constants. Note that, as proved in To infinity — and beyond!, the rule that the derivative of
are constants. Note that, as proved in To infinity — and beyond!, the rule that the derivative of 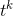 is
is 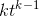 also works for
also works for 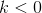 . Show that there is an equilibrium
at
. Show that there is an equilibrium
at 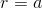 . Verify (either by graphing or by testing the second
derivative) that this is a minimum, not a maximum or a point of inflection.
. Verify (either by graphing or by testing the second
derivative) that this is a minimum, not a maximum or a point of inflection.
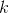 and
and  are constants. Note that, as proved in To infinity — and beyond!, the rule that the derivative of
are constants. Note that, as proved in To infinity — and beyond!, the rule that the derivative of 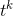 is
is 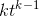 also works for
also works for 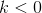 . Show that there is an equilibrium
at
. Show that there is an equilibrium
at 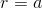 . Verify (either by graphing or by testing the second
derivative) that this is a minimum, not a maximum or a point of inflection.
. Verify (either by graphing or by testing the second
derivative) that this is a minimum, not a maximum or a point of inflection.
- 2452 reads






