Ionic and covalent bonds are strong bonds that require considerable energy to break. However, not all bonds between elements are ionic or covalent bonds. Weaker bonds can also form. These are attractions that occur between positive and negative charges that do not require much energy to break. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. These bonds give rise to the unique properties of water and the unique structures of DNA and proteins.
When polar covalent bonds containing a hydrogen atom form, the hydrogen atom in that bond has a slightly positive charge. This is because the shared electron is pulled more strongly toward the
other element and away from the hydrogen nucleus. Because the hydrogen atom is slightly positive (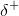 ), it will
be attracted to neighboring negative partial charges (
), it will
be attracted to neighboring negative partial charges (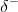 ). When
this happens, a weak interaction occurs between the
). When
this happens, a weak interaction occurs between the 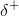 charge of the hydrogen atom of one molecule and the
charge of the hydrogen atom of one molecule and the 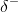 charge of the other molecule. This interaction is called a hydrogen bond. This type of bond is common; for example, the liquid nature of water is caused by the hydrogen bonds
between water molecules (Figure 2.7). Hydrogen bonds give water the
unique properties that sustain life. If it were not for hydrogen bonding, water would be a gas rather than a liquid at room temperature.
charge of the other molecule. This interaction is called a hydrogen bond. This type of bond is common; for example, the liquid nature of water is caused by the hydrogen bonds
between water molecules (Figure 2.7). Hydrogen bonds give water the
unique properties that sustain life. If it were not for hydrogen bonding, water would be a gas rather than a liquid at room temperature.
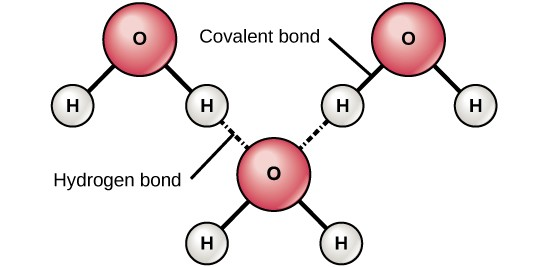
Hydrogen bonds can form between different molecules and they do not always have to include a water molecule. Hydrogen atoms in polar bonds within any molecule can form bonds with other adjacent molecules. For example, hydrogen bonds hold together two long strands of DNA to give the DNA molecule its characteristic double-stranded structure. Hydrogen bonds are also responsible for some of the three-dimensional structure of proteins.
- 3332 reads






